
Software for a smooth-running production
Increase quality and control
Data management for the pharmaceutical and life science industry
Pharmaceutical and life science manufacturers must comply with a range of regulations and standards, such as FDA and GxP. This requires full and detailed documentation of the entire production process and of regularly conducted self-inspections. Each manufacturing step, every organisational measure and all process conditions must be documented end-to-end to provide full records and complete traceability. octoplant help companies adhere to FDA and GxP rules with regard to process quality, production documentation and proof of compliance.
Complete Transparency
octoplant's comprehensive change history allows you to accurately track all production process changes, providing detailed insight into who, what, when and where changes were made.
Compliance Adherence
octoplant facilitates thorough verification of compliance with quality standards, including FDA (21 CFR Part 11) and GxP requirements.
Efficient Release Control
octoplant ensures early error detection and quality assurance by using the four-eye principle within the approval process.







By introducing the AUVESY-MDT solution, we are minimizing the risk of PLC-failures that affect the quality of the medicines. This data management and change-software enables us resolve production stop issues and resume production as quickly as possible.
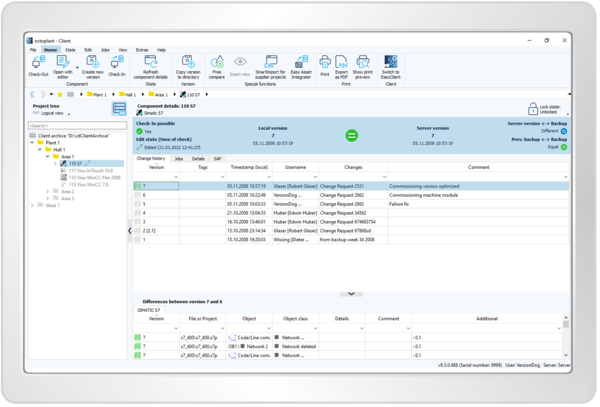
Keep Track of All Devices and Drivers
- Automatically backup assets
- Get a comprehensive 360-degree view of the entire plant
- View a complete change history with information on who made what changes, when, where, and why
- Compare versions graphically and in tabular form
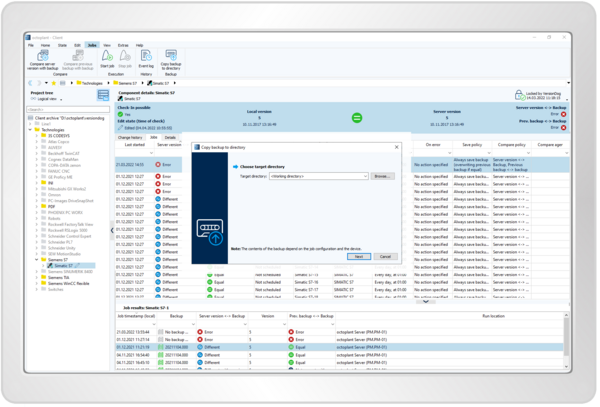
Resume production within minutes for an emergency
- Quickly restore the right system configurations
- Perform pattern-based or custom restores
- Easily organize program backups to see the latest and correct versions
- Alarm function for deviations from set values and parameters

Ensure Regulatory Compliance with Automated Audit Trails
- Document even the smallest project differences
- Audit trails at the touch of a button
- Set-up approval workflow to comply with industry regulations
- Get a detailed risk assessment report for your assets

Get a 360-Degree View of Your Production Environment
octoplant supports more devices than anyone else in the industry, including PLCs (e.g. TIA Portal), SCADA systems, HMIs, and robots from different manufacturers. This hepls you better analyze the shop floor, isolate errors, and fix any problems. See for yourself!


























NIS2’s new security regulations are forcing industry and suppliers to act
Discover the impact of the EU NIS2 Directive on manufacturing companies! With penalties of up to €10 million or 2% of global turnover, stricter cybersecurity requirements are a reality for industries such as pharmaceuticals, chemicals, and more. Our free guide offers concrete solutions to ensure compliance and minimize cyber threats.
Download the free whitepaper now!



