Compliance management: meeting legal and regulatory requirements
Complying with auditing and documentation obligations in a legally secure and comprehensive manner
Integrated documentation and (depending on the industry) regulatory-compliant workflows ensure reliable and seamless compliance management that reduces risk. Be assured the production process complies with regulations, is traceable and, in the event of an audit, can be traced.
This is particularly important for industry-specific requirements. For example, it is vital in process engineering production such as chemicals, pharmaceuticals or foodstuffs, but also in critical infrastructures. Compliance features reduce certification compliance risks, which is subject to different strict international rules. This is particularly important when liability issues need to be clarified in the context of production audits, such as who made which changes and when - data transparency in processing is a must.
CUSTOMER SURVEY
In addition to reducing manual effort, the key advantages of our software solution for our users include streamlining work processes and minimizing downtime and production loss.*
*Source: AUVESY GmbH customer survey, survey period 2023, 162 respondents
Features of octoplant Compliance Management

Define approval processes
Set up approval workflow to comply with industry regulation.

Receive GxP documents (GURs)
Support you in documenting your system validation process for global user requirements (GURs).

What our customers say

The fact that backups can not only be saved, but also compared with each other is an enormous help to us, especially during
audits. The software is set up: all new projects are stored in the solution from AUVESY-MDT as standard. I see no reason as to why we would ever
do without this system again.
Support for quality assurance and documentation requirements
When it comes to compliance with technical specifications, laws, and guidelines such as FDA or GxP, various manufacturing production and process documentation is often required. octoplant provides support for quality assurance, production documentation and rule compliance so that evidence can be provided to monitoring authorities and customers.
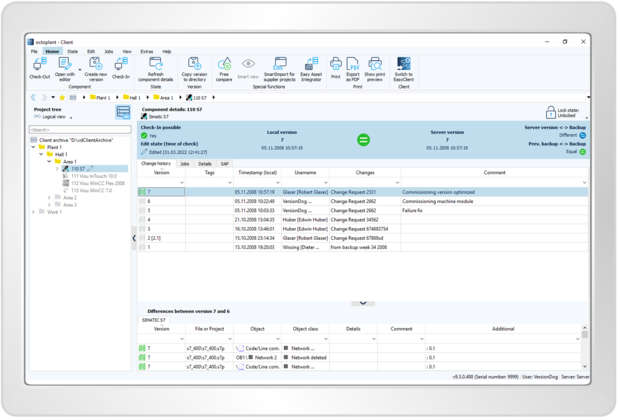
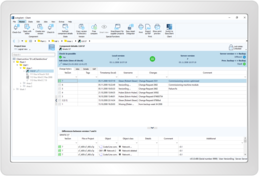
You will receive answers to the typical questions related to process documentation (what, why, who, when, where) – in a standardized and efficient way. Complete data transparency and traceability is produced by audit trail reports at the push of a button, in compliance with the applicable IT laws.
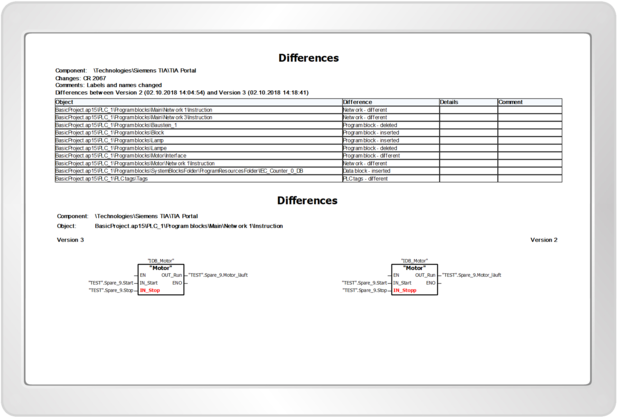
octoplant provides all relevant information needed for audit trail processes to ensure quality assurance and offers a continuous change history and documentation of program changes.

Thanks to the standardized documentation, you will fulfill your documentation requirement and, if desired, be in compliance with the dual control principle. octoplant offers assistance in complying with the ISDS/ISQM standard and allows for customization in meeting FDA and GxP requirements.
Frequently asked questions
octoplant fulfills many of the IT baseline protection requirements for industrial control systems (ICS). octoplant’s key values include prevention, detection, and response in relation to ICS.
octoplant’s electronic signatures meet the requirements of FDA 21 CFR Part 11 and GMP.
If the GxP customer option is activated, another person must approve or confirm a versioning or check-in process by providing their credentials.
Yes. Changes to the programs can be documented using comments when performing a comparison. You can document the changes globally or document each adjustment separately.